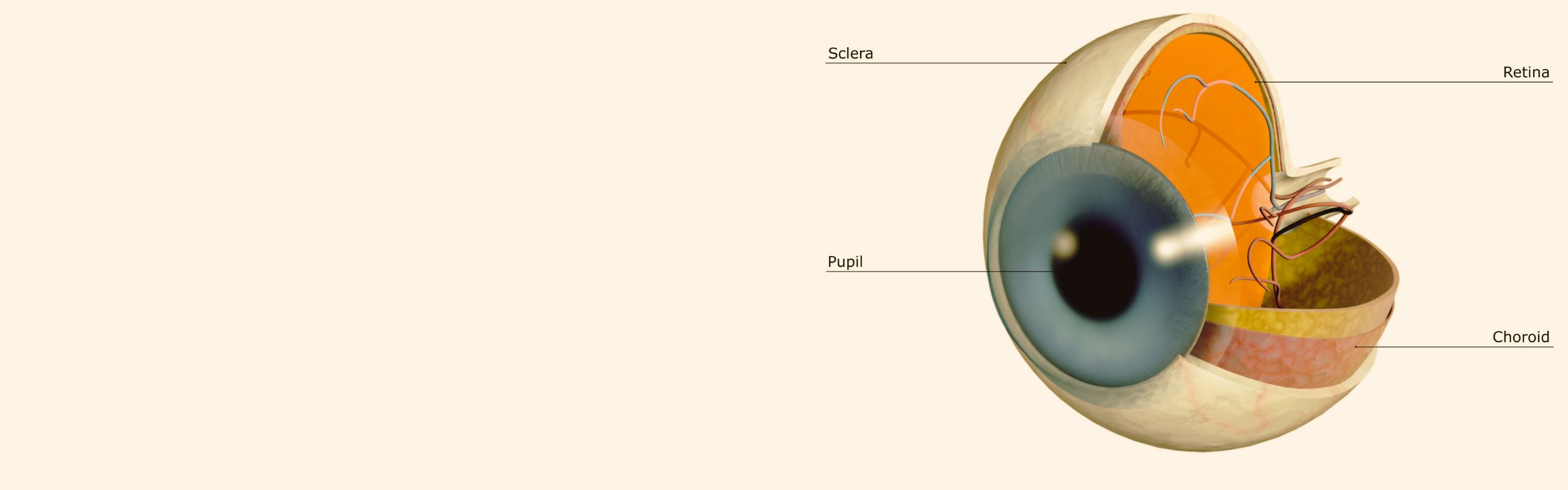
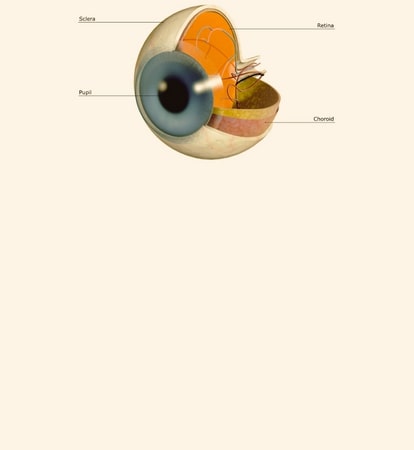
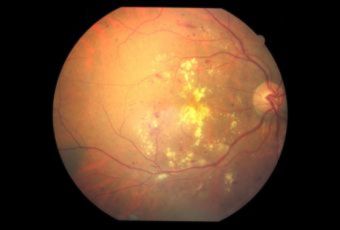
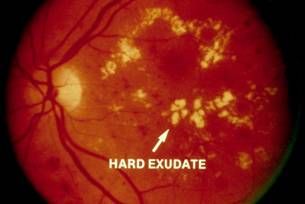
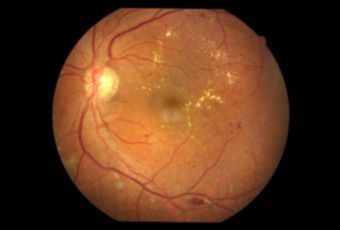
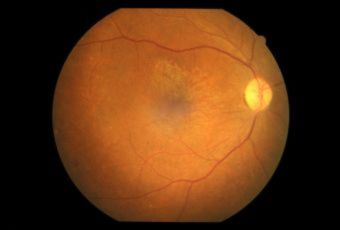
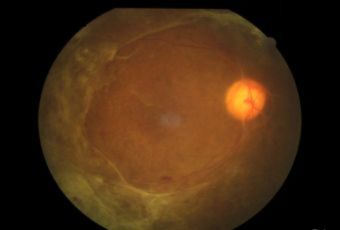
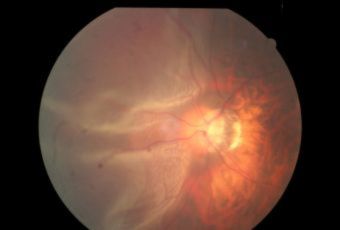
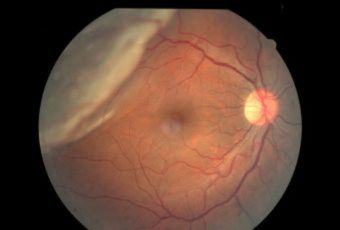
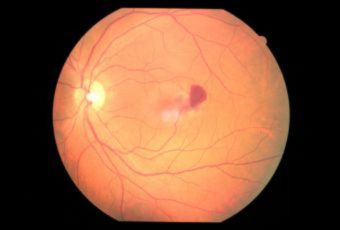
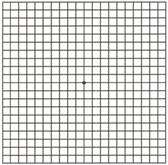
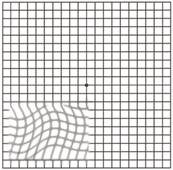
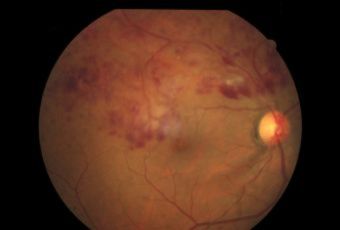
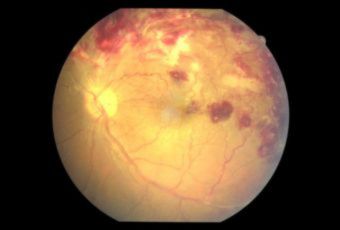
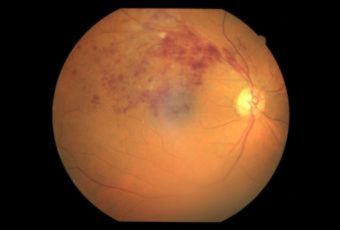
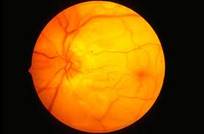
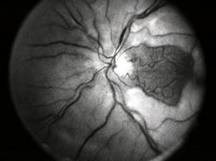
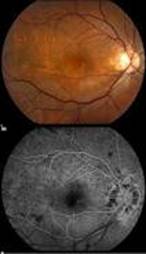
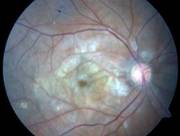
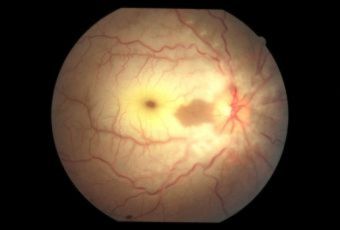
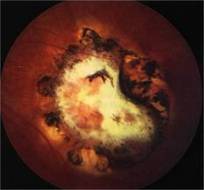
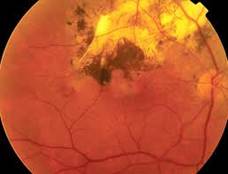
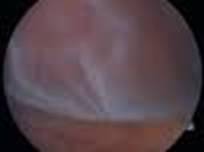
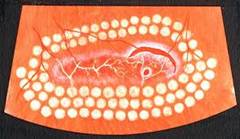
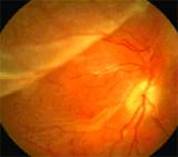
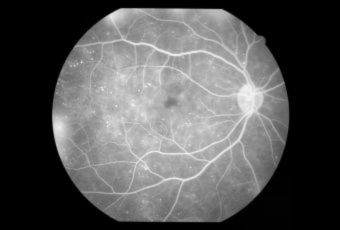
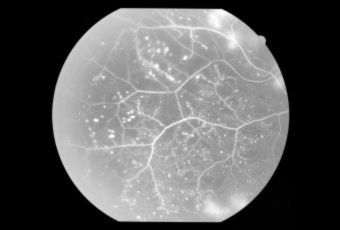
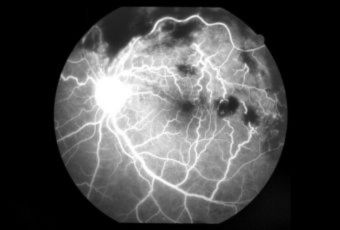
Diabetic Retinopathy |
||
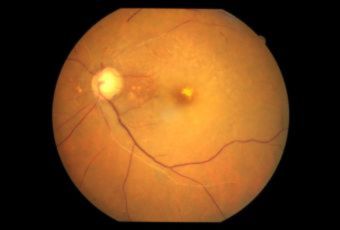
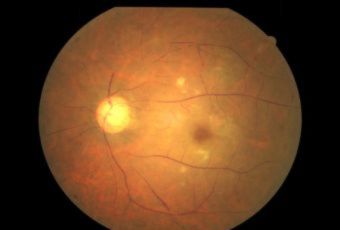
Etiology
Ophthalmic Features
Types of Diabetic RetinopathyThere are two types of diabetic retinopathy:
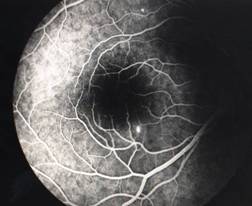
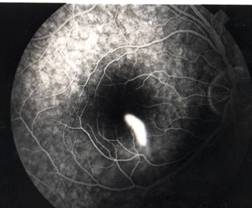
InvestigationsFluorescein Angiography
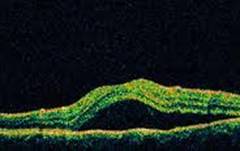
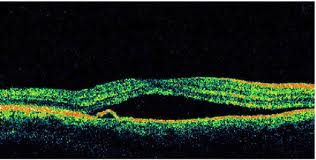
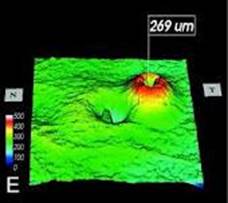
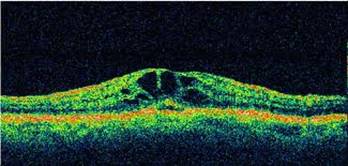
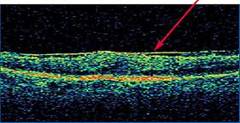
Optical Coherence Tomography (OCT) - In diabetics, OCT can “map” areas of macula edema (“swelling”) thus facilitating fluorescein angiography in guiding laser treatment of the macula. Treatment
|
||
Retinal Detachment |
||
|
Retinal Detachment (R.D.) is a separation of the sensory retina from the retinal pigment epithelium(R.P.E) by sub retinal fluid(S.R.F) There are two main types.
Secondary R.D sub divided in to two types:
Retinal signs depends on duration of R.D.
|
||
AMD |
||
(Vitamin supplements are not cures for AMD, nor can they restore vision already lost from AMD.)
|
||
BRANCH RETINAL VEIN OCCLUSION (BRVO) |
||
|
||
CENTRAL RETINAL VEIN OCCLUSION (CRVO) |
||
|
The actual mechanisms producing the clinical picture of central retinal vein occlusion may be roughly divided into those conditions that produce a physical blockage at the level of the lamina cribrosa, and those conditions in which hemodynamic factors result in an obstruction to the flow of blood. These mechanisms probably coexist in many patients with Central Vein Occlusion.
Confluent hemorrhages are the most prominent ophthalmoscopic feature of an acute ischemic central retinal vein occlusion These hemorrhages occur in a wide variety of shapes and sizes; they are usually concentrated in the posterior pole, but may be seen throughout the retina. Many hemorrhages are flame shaped, reflecting the orientation of the nerve fibers. Dot and punctate hemorrhages are interspersed and indicate involvement of the deeper retinal layers. Bleeding may be extensive, erupting through the internal limiting membrane to form a preretinal hemorrhage or extending into the vitreous. Small dot hemorrhages may be seen either isolated or clustered around small venules. The entire venous tree is tortuous, engorged, dilated, and dark. The retina is edematous, particularly in the posterior pole; some of this edema may obscure portions of the retinal vessels. Cotton-wool patches (soft exudates) are often present. The disc margin is blurred or obscured, and the precapillary arterioles appear engorged. Splinter hemorrhages and edema are present on the disc surface and extend into the surrounding retina. The physiologic cup is filled, and the venous pulse is absent. The arterioles, often overlooked because of the other more striking pathologic features, are frequently narrowed. Sometimes in central retinal vein occlusion of acute onset, the fundus picture is less dramatic, and all of the findings previously discussed may be present, but to a lesser degree. Vision depends on extent of macular involvement
|
||
CENTRAL RETINAL ARTERY OCCLUSION (CRAO) |
||
|
||
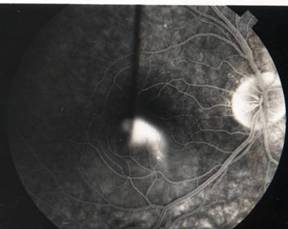
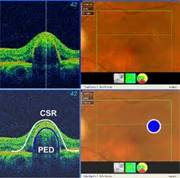
CENTRAL SEROUS RETINOPATHY (CSR) |
||
Smoke stack appearance
Ink blot appearance
|
||
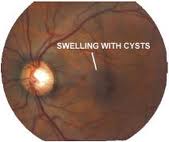
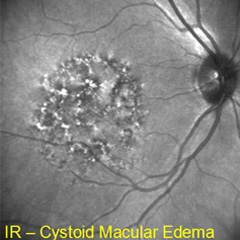
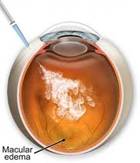
MACULAR EDEMA |
||
Etiology
Symptoms
Treatment
Preventive Treatment
Medical Treatment
Surgical Treatment
|
||
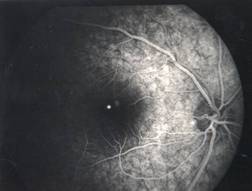
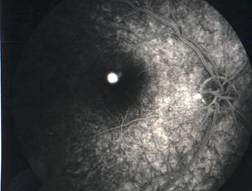
MACULAR HOLE |
||
Etiology
Ophthalmic features
Investigations
|
||
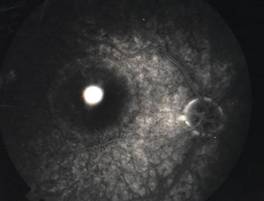
MACULAR PUCKER |
||
Etiology
Ophthalmic features
Treatment
|
||
RETINITIS PIGMENTOSA |
||
|
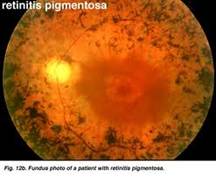
Retinitis pigmentosa is the most common of a group of hereditary progressive retinal degenerations or dystrophies. There is considerable variation and overlap among the various forms of retinitis pigmentosa. Common to all of them is progressive degeneration of the retina, specifically of the light receptors, known as the rods and cones. The rods of the retina are involved earlier in the course of the disease, and cone deterioration occurs later. In this progressive degeneration of the retina, the peripheral vision slowly constricts and central vision is usually retained until late in the disease. Etiology
Ophthalmic features
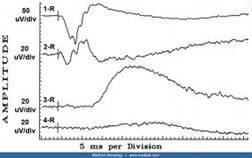
Diagnosis
Treatment
|
||
TOXOPLASMOSIS |
||
|
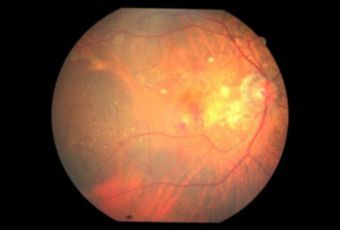
Toxoplasmosis is a disease provoked by the obligate intracellular protozoan Toxoplasma gondii. It is found in a variety of mammal and bird hosts. The most common intermediate host is the cat. It is one of the most frequent causes of retinochoroiditis in humans, with more than 60 percent of the United States population and up to 75 percent of the world's general population possessing some seropositive findings Etiology
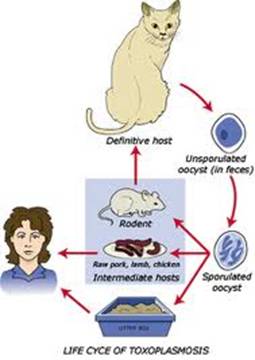
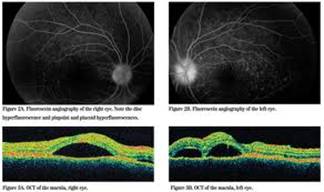 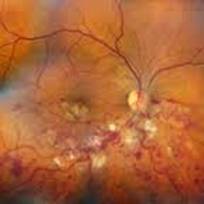
Ophthalmic Features
Treatment
|
||
LATTICE DEGENERATION |
||
|
Lattice degeneration is a disease of the eye where peripheral retina becomes atrophic in a lattice pattern and may develop tears / breaks / holes, which may further progress to retinal detachment. It is an important cause of retinal detachment in young myopic individuals. The cause is not known but pathology reveals vascular insufficiency resulting in ischemia and fibrosis. Etiology
Ophthalmic features
Treatment
|
||
FLOATERS & FLASHES |
||
Etiology
Ophthalmic Features
Treatment
|
||